Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – USA News Group News Commentary – As the Trump administration signals potential cuts to the National Institutes of Health (NIH), questions are beginning to surface about how future cancer research efforts will be impacted at the federal level. In response, private initiatives are gaining traction, including a new immunotherapy-focused cancer center spearheaded by former Citi CEO Sandy Weill in partnership with four major research institutions. Meanwhile, a more positive outlook is taking shape in Europe, where projections from a University of Milan and University of Bologna study suggest cancer mortality rates may decline this year. At the forefront of therapeutic innovation, several oncology companies have already seen significant activity in early 2025, including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Summit Therapeutics Inc. (NASDAQ: SMMT), Immatics N.V. (NASDAQ: IMTX), Candel Therapeutics, Inc. (NASDAQ: CADL), and Exelixis, Inc. (NASDAQ: EXEL).
The article continued: According to Statista, the number of annual cancer cases is expected to rise by 20% by 2030 and jump by 75% by 2050. A recent report from Vision Research Reports estimates that the global oncology market will exceed US$903.81 billion by 2034, driven by a compound annual growth rate of 10.9%.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage company focused on immunotherapy for cancer, highlighted today how the company and its immunotherapeutic agent pelareorep were discussed by field-leading key opinion leaders (KOLs) during a recent event hosted by H.C. Wainwright—a replay of the full conversation can be found by clicking here.
Martine Piccart, M.D., Ph.D., an Honorary Professor of Oncology at the Université Libre de Bruxelles (ULB) and Scientific Director of Oncology at the Institut Jules Bordet, in Brussels, Belgium, offered a detailed overview of the HR+/HER2- metastatic breast cancer landscape and emphasized the need for new treatment innovations, such as pelareorep, that work to activate the immune system to recognize and kill cancer.
Alexander Eggermont, M.D., Ph.D., Professor of Clinical & Translational Immunotherapy at the University Medical Center Utrecht in the Netherlands and Board Member of the Comprehensive Cancer Center Munich of the Technical University Munich and the Ludwig Maximilians University, Munich, Germany, provided insights on the current standards for treating pancreatic ductal adenocarcinoma (PDAC), a cancer type known for its resistance to treatment, and the potential impacts that an immunotherapy such as pelareorep might have on the field.
Over the past several months, Oncolytics Biotech has remained focused on advancing its lead candidate, pelareorep, an intravenously delivered immunotherapy being tested across multiple cancer types. The company’s research continues to concentrate on three areas where treatment options are limited: metastatic breast cancer, pancreatic cancer, and anal cancer.
“With multiple clinical trials surpassing expectations in 2024, 2025 is shaping up to be a defining year for Oncolytics,” said Wayne Pisano, Chair of Oncolytics’ Board of Directors and Interim CEO. “Our top priority is HR+/HER2- metastatic breast cancer, in which two randomized trials involving over 100 patients have shown substantial clinical benefit for patients receiving pelareorep and paclitaxel compared to paclitaxel monotherapy. We believe that if we can approximate the benefit we saw in BRACELET-1 in our planned registrational study, the progression-free survival benefit alone would support an accelerated approval submission.”
Oncolytics Biotech is advancing pelareorep, its lead immunotherapy candidate, in metastatic breast, pancreatic, and anal cancers. In the BRACELET-1 Phase 2 breast cancer trial, pelareorep plus paclitaxel outperformed chemotherapy alone, supporting plans for a larger study in 2025 that could lead to accelerated approval.
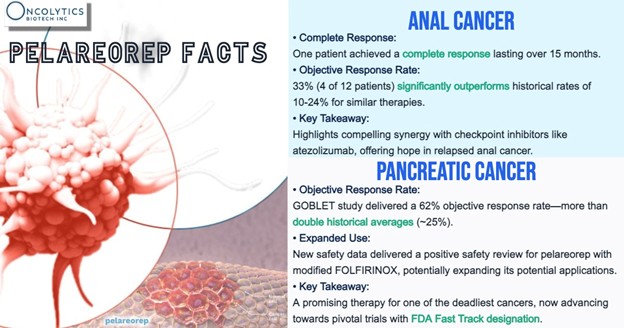
In pancreatic cancer, the company is enrolling patients in a cohort of the GOBLET study using pelareorep with modified FOLFIRINOX, with or without atezolizumab, following safety clearance from German regulators. Initial efficacy results are expected later this year.
In anal cancer, pelareorep combined with atezolizumab showed a 33% response rate, including a complete response lasting over 15 months, prompting an expansion in enrollment to confirm the efficacy signal and potentially move this treatment regimen to a registration-enabling study.
As of year-end 2024, the company held $15.9 million in cash, with a runway into Q3 2025.
While the path forward includes typical biotech risks, Oncolytics has moved beyond early-stage development and now has clinical data that may support future regulatory discussions. With multiple studies and several updates expected over the coming months, 2025 is shaping up to be a year of continued clinical progress.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Summit Therapeutics Inc. (NASDAQ: SMMT) recently appointed Robert LaCaze as its new Chief Commercial Officer to help lead the potential launch of its cancer drug candidate, ivonescimab. LaCaze brings over 35 years of experience in oncology, including leadership roles at Bayer and Bristol-Myers Squibb, where he guided major immuno-oncology franchises.
"Summit's commitment to excellence and its highly-accomplished team provide me with great confidence about what can be achieved with such an innovative, novel product candidate, ivonescimab,” said LaCaze. “I look forward to working with the team to help drive a successful commercial launch of ivonescimab and the potential to make a meaningful impact in the lives of patients with cancer."
Immatics N.V. (NASDAQ: IMTX) in its recently released FY 2024 financial results and business update highlighted how it is now enrolling patients in its global Phase 3 trial for IMA203, a TCR-T therapy for advanced melanoma, following promising Phase 1b data showing a 54% confirmed objective response rate and strong durability. The company aims to submit for full approval in 2027 and is building commercial manufacturing capacity to support launch.
In parallel, Immatics is progressing its next-generation cell therapy IMA203CD8 and two bispecific candidates (IMA402 and IMA401), targeting cancers such as ovarian, head and neck, and NSCLC. Backed by over $600 million in cash, Immatics expects key updates across its pipeline throughout 2025.
Candel Therapeutics, Inc. (NASDAQ: CADL) recently announced strong survival outcomes from its Phase 2a trial of CAN-2409 in patients with advanced NSCLC who did not respond to prior immunotherapy. Median overall survival reached 24.5 months in the full cohort, and 21.5 months in patients with progressive disease at enrollment—well above survival expectations for this population.
“The extension of survival in patients with non-squamous disease is notable even when compared to data that have been reported for other investigational products, such as antibody-drug conjugates, for this patient population,” said W. Garrett Nichols, MD, CMO of Candel. “CAN-2409, in addition to continued ICI treatment, may prolong survival beyond that offered by docetaxel chemotherapy, and has the potential to be better tolerated.”
The treatment demonstrated a long tail of survival, with over one-third of patients still alive two years after therapy. Backed by favorable safety and biomarker data, Candel is advancing CAN-2409 toward a potential registrational trial in non-squamous NSCLC.
Exelixis, Inc. (NASDAQ: EXEL) recently announced that CABOMETYX® has been approved by the FDA for patients with previously treated advanced pancreatic and extra-pancreatic neuroendocrine tumors. The approval follows strong Phase 3 results from the CABINET trial, which showed meaningful improvements in progression-free survival.
"As a company committed to improving the standard of care for people living with advanced, difficult-to-treat cancers, we are proud to bring CABOMETYX to patients with previously treated advanced neuroendocrine tumors," said Amy Peterson, M.D., Executive Vice President, Product Development & Medical Affairs, and Chief Medical Officer, Exelixis. "I would like to extend our sincere gratitude to the Alliance for Clinical Trials in Oncology for conducting the CABINET trial, to the FDA for their collaboration on the review of this application and to all the patients and physicians who participated in this important study. Looking forward, we are doubling down on our commitment to the NET community as we prepare to initiate our STELLAR-311 pivotal trial examining zanzalintinib versus everolimus in the first half of 2025."
This is the first systemic therapy approved for NETs regardless of primary tumor site, grade, somatostatin receptor expression and functional status. According to the press release, Exelixis is prepared to immediately support these new indications.
Source: https://usanewsgroup.com/2024/09/21/is-oncolytics-biotech-the-markets-most-undervalued-cancer-opportunity/
CONTACT:
USA NEWS GROUP
[email protected]
(604) 265-2873
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice commencing immediately and ongoing. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material, including this article, which is disseminated by MIQ has been approved by Oncolytics Biotech Inc.; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.