A little-known biotech company is poised to potentially earn millions in revenue with the planned upcoming release of a breakthrough technology that could prevent massive numbers of strokes with a simple test that is affordable and accessible to the average American.
A Multi-Billion-Dollar Problem with a $49,000 Answer
Stroke statistics are shocking: Globally, every year 15 million people suffer a stroke. Of these, some 6 million are killed, while 5 million are rendered permanently disabled. In the U.S. alone, nearly 800,000 people suffer from strokes annually—and someone dies of a stroke every 4 minutes.
Until now, there has been no cost-effective way to screen for Ischemia, the leading indicator of a stroke, which cost the U.S. government alone tens of billions of dollars a year. That could be about to change, thanks to a breakthrough technology from CVR Medical (TSX:CVM.V ; OTC:CRRVF. The company’s debut, game-changing medical device has been quietly in development for 10 years, and now it’s about to charge out of the gate, hoping to take the market by storm upon FDA market clearance.
What 3D seismic imagery did for super quick discoveries in the oil and gas industry, CVR’s sensory system could do for the medical industry.
CVR’s Carotid Stenotic Scan (CSS) is a tool to detect stenosis within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner. Unlike other comparative modalities, the CSS was designed to function without the assistance of a certified technician. These three facts combine to create one of the potentially biggest—and most lucrative--phenomena in recent medical equipment market history.
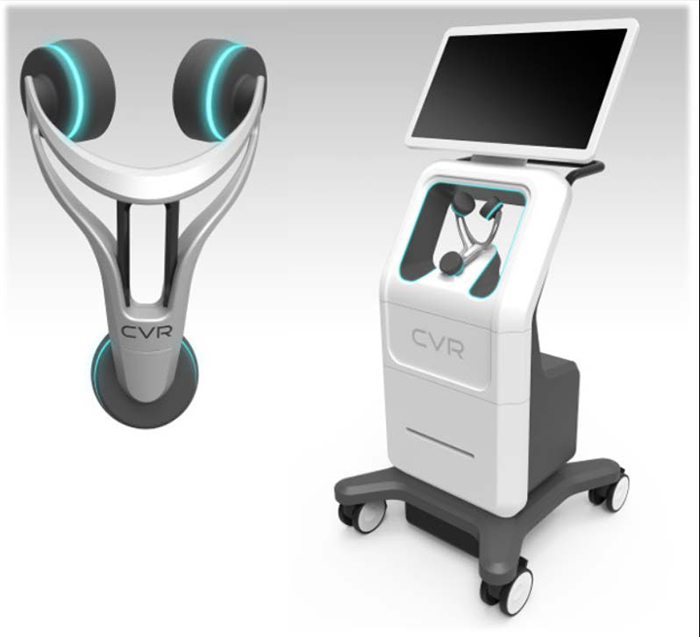
The CSS makes a connection between fluid flow and sub-sonic frequencies to detect arterial disease or blockage. Blood flowing through the carotid arteries produces wave patterns which are shaped and altered by the presence of irregularities on the inner artery walls. CVR’s advanced technology captures these wave patterns and analyzes them mathematically with patented algorithms. After a brief test, the analysis is complete, offering a way to potentially identify those at risk of a stroke and arming the healthcare provider with the information necessary to prevent the deadly event. For investors, medical providers, insurers, and the public at large, the secondary benefit is that this new innovation has a price tag that renders testing up to 50 times cheaper than many tools on the market today.
Coming in at $49,000 per unit, compared to up to a whopping US$2.5 million for existing technology.
Additionally, some existing technologies are invasive and, due to cost, not accessible to many. CVR’s CSS is positioned to potentially fill this current gap. Once available, the CSS technology stands to save the U.S. government up to US$34 billion a year, the current cost of strokes annually in the U.S. according to the CDC.
With strokes coming in as the second leading cause of death globally for people over the age of 60, and the fifth leading cause of death for people aged 15-59, a new technology that can deliver early detection is beyond critical.
According to the CDC, early action is urgent for survival, and only 38 percent of stroke sufferers even recognize they are having a stroke in time to receive effective emergency intervention.
2017: The Definitive Year of Biotech
This is where the new barons are being made, with medical breakthroughs turning countless billions in new profits—and 2017 is set to be the strongest ever.
- Biotech companies pivoting to microbiome to develop new diagnostics, new therapies and probiotic products to prevent dangerous microbe imbalances are set to cement themselves as one of the industry’s most promising and lucrative “frontiers” this year.
- Cellular immunotherapies reporting 90 percent remission rates for acute lymphoblastic leukemia (ALL) is expected to be presented to the FDA in 2017 and could trigger a wave of approvals for other blood cancers and lymphomas, and could eventually replace chemotherapy.
- Liquid biopsies, or blood tests that uncover signs of actual DNA or cell-free circulating tumor DNA (ctDNA), could prove to be a revolutionary cancer test with annual sales forecast to be $10 billion.
- Bioabsorbable stents, approved in the U.S. in July for the first time, has a market potential approaching $2 billion in six years, with this year the critical juncture.
- CVR invested $23 million in its breakthrough technology, with early stage clinical trials complete and now headed toward pivotal trials, eyeing the potential for immediate profitability, with possible U.S. sales alone up to 400,000 devices annually at $49,000 each. When you talk globally, the numbers are staggering.
There’s nothing more gratifying than getting in on the ground floor of a medical equipment breakthrough that can save millions of lives and turn a fantastic profit while doing it.
Here are 3 reasons to keep a close eye on CVR Medical right now:
1) Urgent Need, Guaranteed Market
Few things are more urgent that an early detection system for a medical condition that kills 6 million people a year. In the U.S. alone, one American dies from stroke every 4 minutes, and right now the U.S. death toll from strokes stands at upwards of 130,000 each year. Annually, more than 795,000 Americans suffer a stroke.
“Strokes will absolutely strain the healthcare system,” said Bruce Ovbiagele, M.D., M.Sc., professor and chairman of the Department of Neurology at the Medical University of South Carolina, Charleston. Caring for survivors is expensive because stroke can cause long-term disability, he said. “Ninety percent of stroke patients have residual disability and only 10 percent recover completely after a stroke,” Ovbiagele said. “Policy makers at all levels of governance should be aware of this looming crisis so that we can consider practical ways to avert it.”
Against the backdrop of these devastating statistics, CVR is poised to make a dramatic impact upon an industry starved for innovation and advancement.
The CSS is a groundbreaking tool that can assess Carotid Arterial health in a way that has never been available to a patient, healthcare provider, or the payor in our current healthcare system. Existing early detection systems cost anywhere between US$250,000 and US$2.5 million—and they aren’t economically feasible to use, so the indicators for a stroke go undetected and the concomitant medical bills continue to drown patients and government alike, while people suffering when it could be prevented.
From the 234,615 primary care offices, specialist offices and hospitals and clinics just in the U.S., the CSS has already identified a total U.S. market opportunity of $11.5 billion, assuming only one device is sold to each office. The global market, then, is gargantuan—and CVR expects to fully penetrate it.
2) The Dream Team Behind the Medical Miracle
The first thing that comes to mind when you meet the team behind CVR and the development of this breakthrough technology is an unheard-of modesty and professionalism. This team has demonstrated the strategic vision of a supergiant by leveraging intellectual property, market and industry know-how and key strategic relationships.
Led by Chairman, CEO and President Peter Bakema—with an impressive 30-year track record in business development, since its inception, CVR has brought on some of the most respected medical professionals in the industry.
- Tony Robinson, COO and Executive Vice-President has been with CVR for 8 years and has extensive domestic and global healthcare experience.
- Michael Rhodes, VP of Quality Systems, is a former VP for Quality for HSBC and Motorola. He has 20 years of experience in multiple markets.
- Dr. W. Douglas Weaver, a member of the BOD Scientific Advisory Board, is the former president of the American College of Cardiology and the former VP and System Medical Director of Heart and Vascular Services at Henry Ford Health System. His over 330 publications related to drug and device discovery have been some of the most influential in our time.
Together, they are on a trajectory which will revolutionize healthcare by offering easily accessible, affordable early detection for a potentially massive market share at a very critical time in our healthcare story.
3) Cashed Up, but Still Cheap and Ready to Burst through the Gates
CVR has already invested US$23 million into the research and development of their breakthrough CSS technology, and right now they’re at that critical juncture where investment turns into profit, so shares are still cheap—while they last, and that isn’t likely to be for long.
CVR is just coming out of early stage clinical trials, and is about to enter pivotal trials, which limits the window of opportunity between market and profitability. They’ve been in business for a decade, and now at the last stage before profitability.
The critical timeframe to watch is the release of a preliminary clinical report in the near future followed by a full clinical report roughly 4-8 weeks later. Once that happens, the next step is FDA submission to prove safety and efficacy, and if successful, market clearance and delivery to the market.
Because the all-in costs are less than half the sale costs, the company is expecting a very quick and lucrative head start. The team has already lined up manufacturing and components, so once the clinical reports are in, and the FDA hurdle is cleared, it’s breakout time.
But it won’t stop here. This isn’t a one trick pony. CVR’s CSS is only the first in the application of the technology.
Soon enough, you could see these Carotid Stenotic Scan (CSS) in every doctor’s office, lab, hospital you visit...
With a potential market of some 235,000 medical offices, we’re looking at an immense market opportunity.
And this company is at the center of it all.
Legal Disclaimer/Disclosure: This piece is an advertorial and has been paid for. This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment. No information in this Report should be construed as individualized investment advice. A licensed financial advisor should be consulted prior to making any investment decision. We make no guarantee, representation or warranty and accept no responsibility or liability as to its accuracy or completeness. Expressions of opinion are subject to change without notice. We assume no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission. Furthermore, we assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information, provided within this Report.